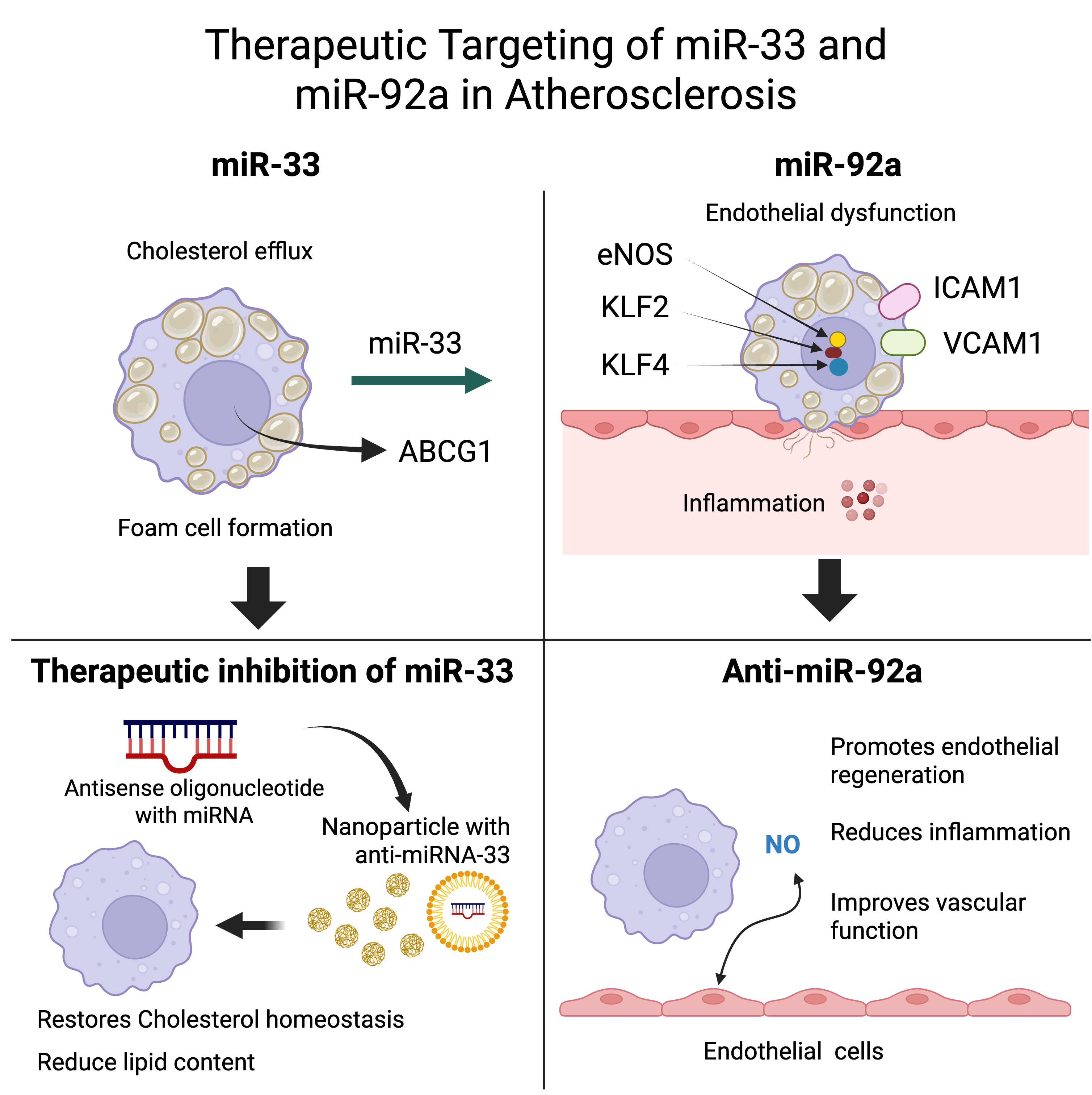
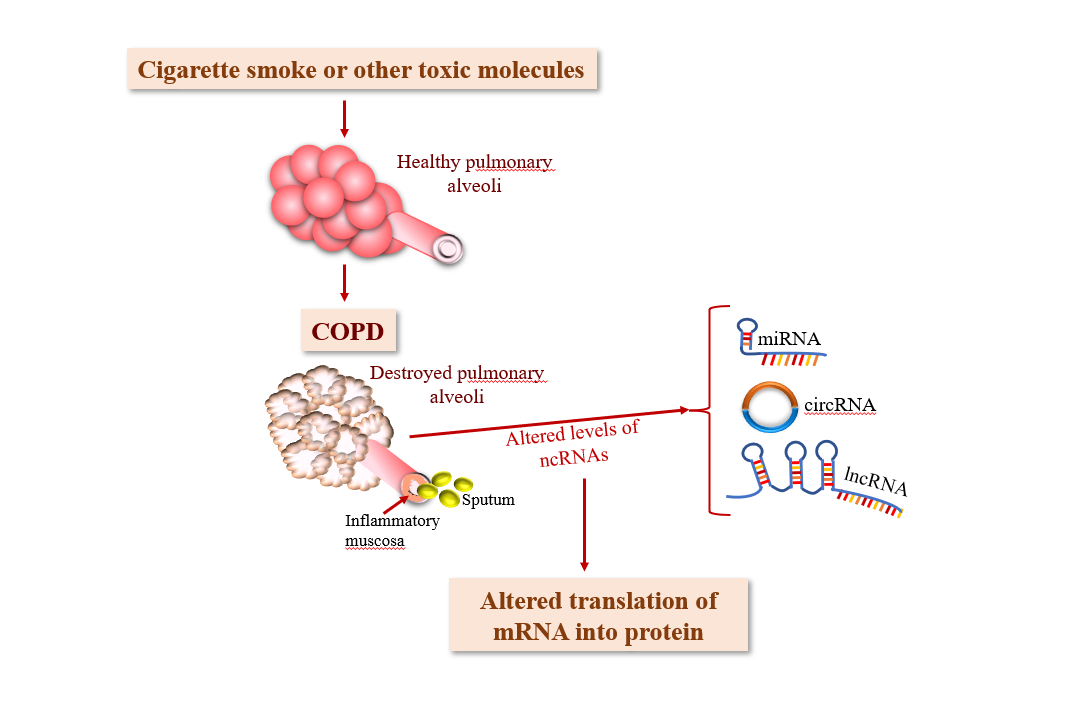
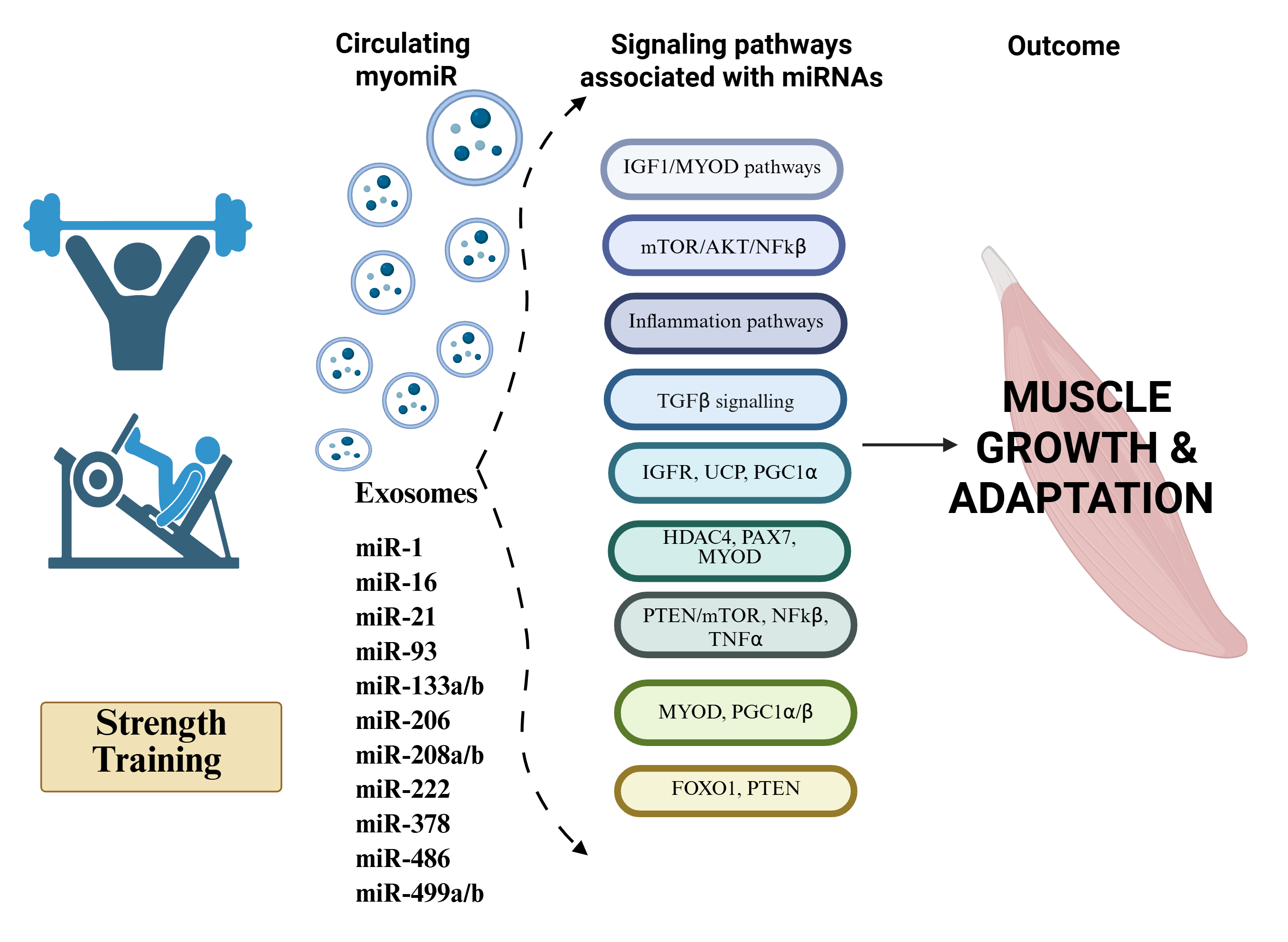
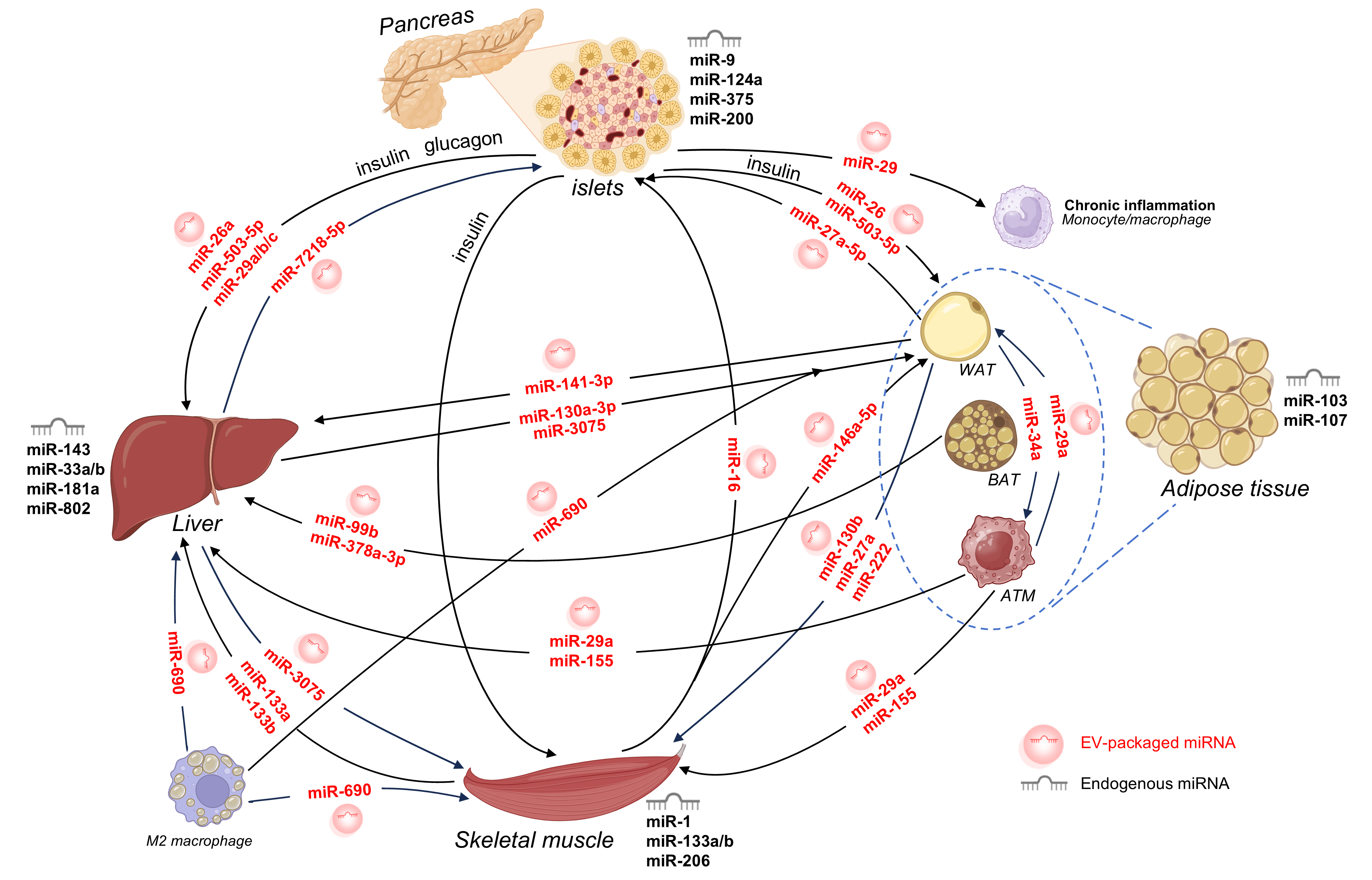
Volumes & Issues
Contact
For any inquiries regarding journal development, the peer review process, copyright matters, or other general questions, please contact the editorial office Ms. Ada Gu, E-Mail: exrna@elspub.com.
For production or technical issues, please contact the production team, Mr. Jay Zhuang, E-Mail: production@elspub.com.
About This Journal
Latest Articles
Editor's Choice
Top Downloaded
Exosomes: a potential biomarker and therapeutic targets in diabetic cardiomyopathy
Published: 22 Oct, 2024