Volumes & Issues
Contact
For any inquiries regarding journal development, the peer review process, copyright matters, or other general questions, please contact the editorial office.
Editorial Office
E-Mail: neuroelectronics@elspub.com
For production or technical issues, please contact the production team.
Production Team
E-Mail: production@elspub.com
About This Journal
Special Issues
Silicon-Based Probes for Invasive Brain-Computer Interfaces: Engineering Innovations and Clinical Translation
Special Issue Editor: Chen Liu, Minghao Wang
Submission Deadline: 30 June 2026
Latest Articles
Editor's Choice
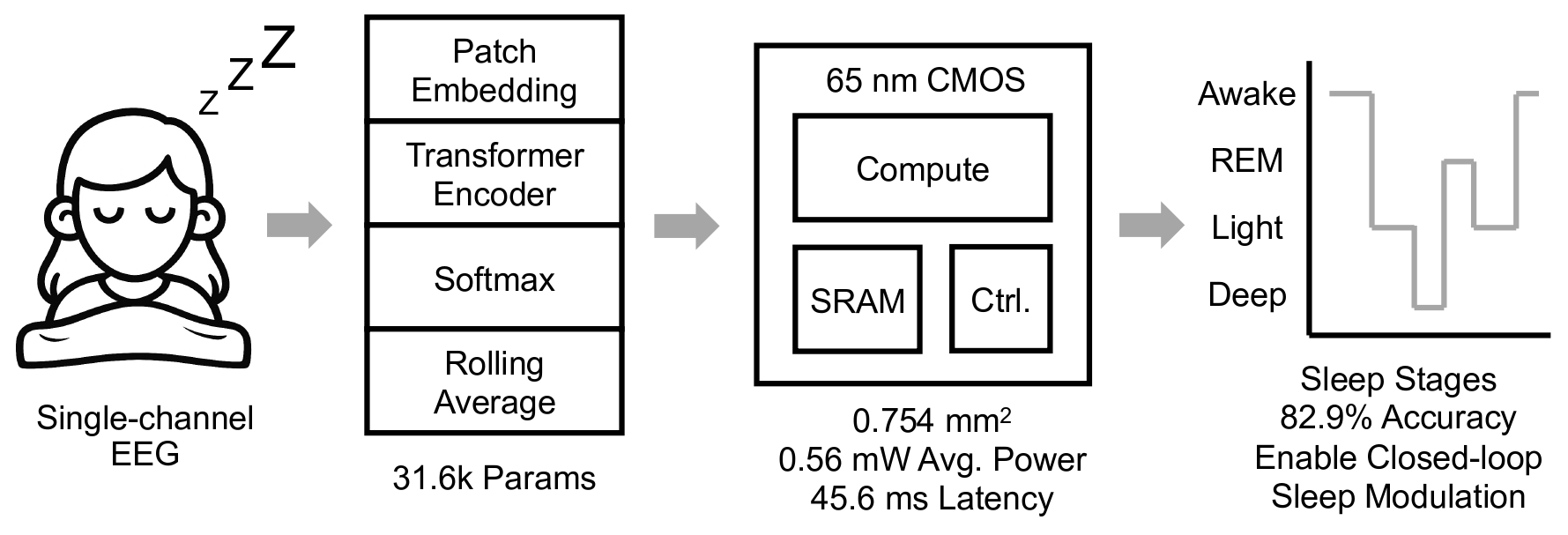
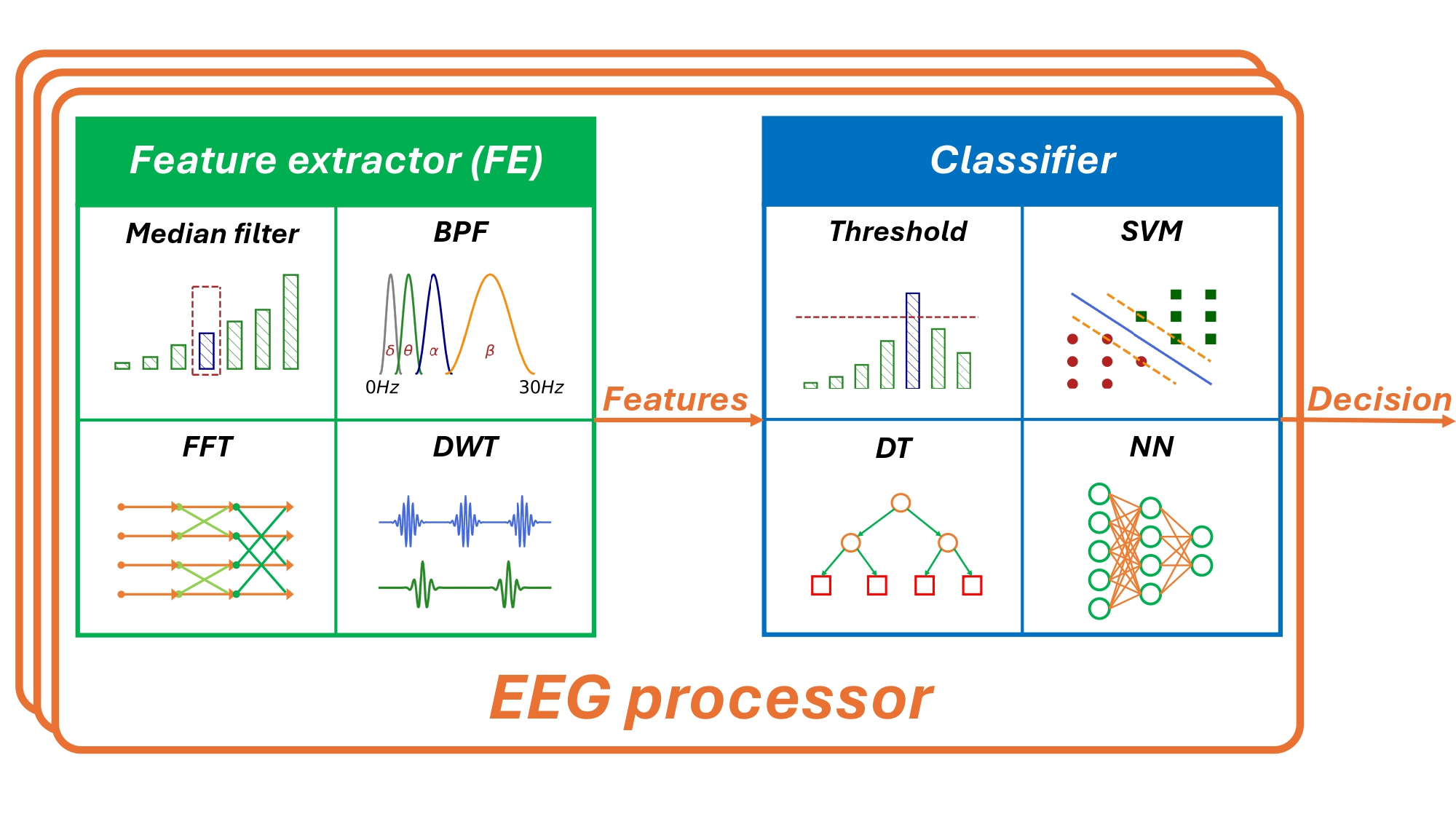
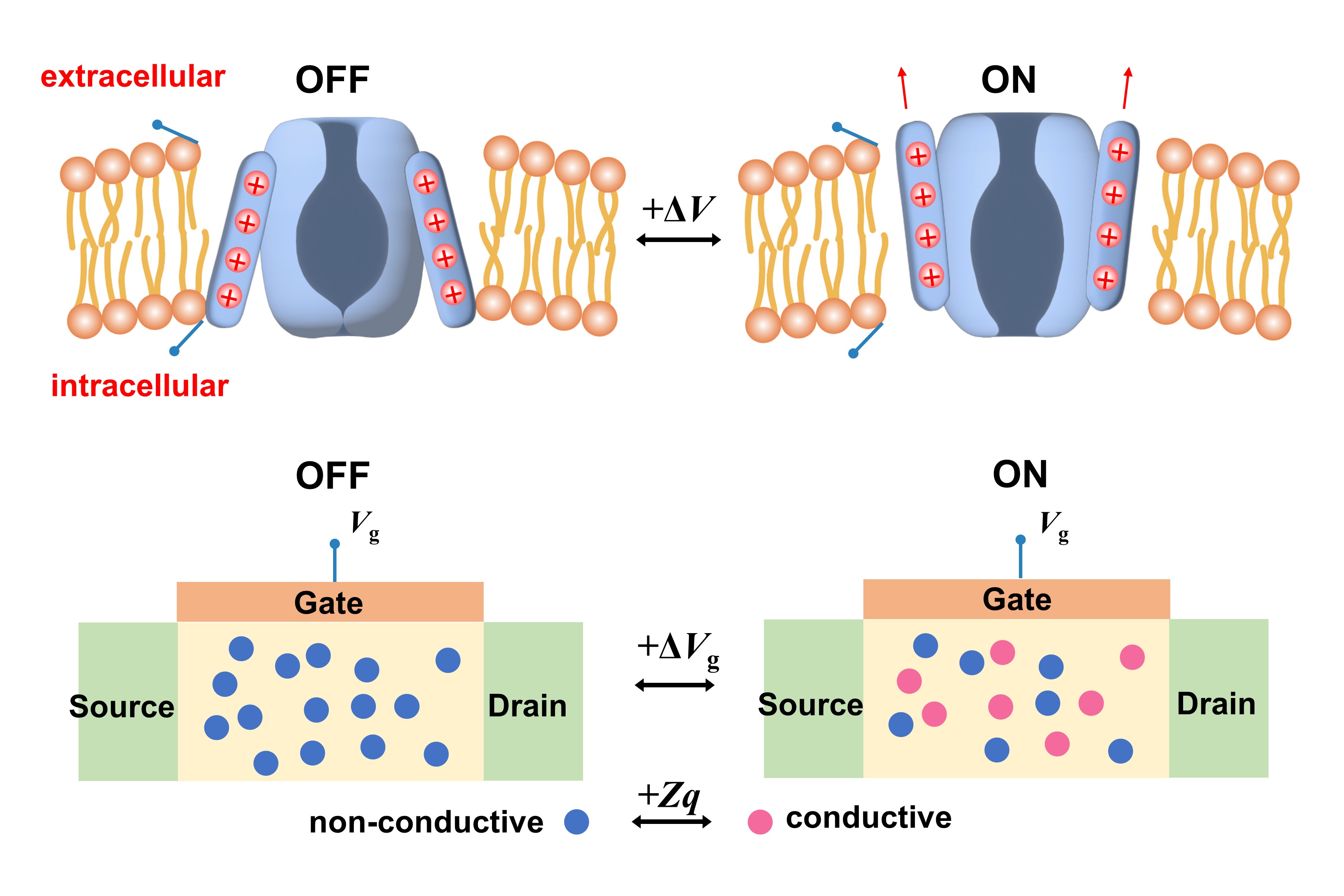
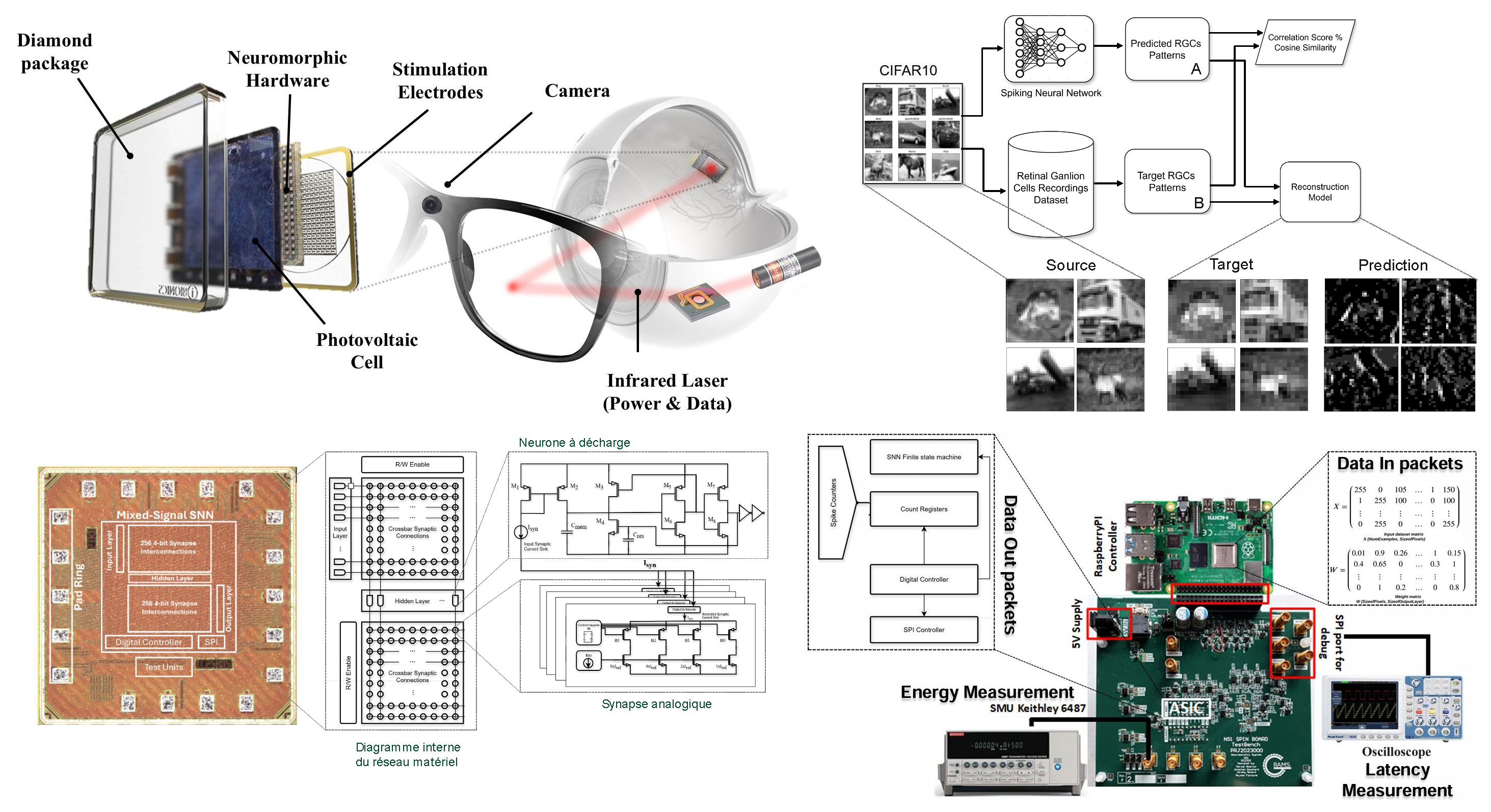
Energy-efficient spiking neural network implementation for a retinal prosthesis
Published: 14 Apr, 2025
Top Downloaded